difference between gravimetric precipitation and volatilization method|example of volatilization gravimetry : service In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate .
Resultado da 3 de dez. de 2021 · Benfica-Sporting, jogo da 13.ª jornada Liga Bwin, no Estádio da Luz (1-3). DIRETO. 90'+7' Termina o dérbi no Estádio da Luz. Benfica-Sporting, 1-3. 90'+7' Nuno Santos arranca pela esquerda, cruza atrasado e Paulinho, na passada, remata ao lado. Golo: 1-3
{plog:ftitle_list}
Resultado da 『美少女戦士セーラームーン』連載開始から30年の軌跡をたどる大展覧会。今回の展覧会のために原作者・武内直子氏が描き下ろした新作イラストや、初公開展示を含む貴重なカラー原画を3期間に渡って過去最大規模で展示しま .
Unlike precipitation gravimetry, which is rarely used as a standard method of analysis, volatilization gravimetric methods continue to play an important role in chemical analysis. Several important examples are discussed below.The best way to appreciate the theoretical and practical details discussed in this .Precipitation and volatilization gravimetric methods require that the analyte, or . Gravimetric Methods: Precipitation involves forming a solid, while volatilization involves converting a solid to a vapor. Precipitation vs. Coprecipitation : Precipitation forms a .
Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. In some situations, .
In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate .8A.2 Types of Gravimetric Methods. The four examples in the previous section illustrate diferent ways in which the measurement of mass may serve as an analytical signal. When the signal is . Volatilization gravimetry involves separating out volatile compounds and measuring the change in mass. Using volatilization gravimetry, we can determine the mass of .Contents. Steps in Gravimetric Analysis. Precipitation Agents. Mechanism of Precipitation. Impurities of Particles. Ways to Minimize Impurities. Gravimetric Calculations. Volatilization .
The principle of gravimetric analysis is based on the estimation of the mass percent of an ion in an impure compound of known quantity by determining the mass of the same ion in a pure compound. In order to determine the mass, . Some authors distinguish two sorts of gravimetry by precipitation: direct gravimetry, in which the analyte itself is weighed. For example, when nickel is determined by .[1] The four main types of this method of analysis are precipitation, volatilization, electro-analytical and miscellaneous physical method. [2] . The methods involve changing the phase . The difference between the original weight and the weight after drying equals the mass of water lost. . and precision of a gravimetric volatilization method is similar to that described in the last section for .
best iphone x drop test
Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. . When the signal is the mass of a precipitate, we call the method precipitation gravimetry. Unlike precipitation gravimetry, which rarely is used as a standard method of analysis, volatilization gravimetric methods continue to play an important role in chemical analysis. . The difference between the original weight and the weight after drying equals the mass of water lost. . Volatilization gravimetric methods are time and labor .The main difference between colloidal precipitate and crystalline precipitate is that colloidal precipitates do not form easily and are difficult to be obtained via filtering whereas crystalline precipitates are easily formed and are easily obtained via filtering. (b) a gravimetric precipitation method and a gravimetric volatilization method.
The gaseous molecules are usually liberated from the solid quite easily because they are volatile and can be evaporated relatively easily. Volatilization gravimetry differs from other gravimetric analysis methods that use precipitation or electricity to separate the components of a mixture or chemical substance.Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. . Precipitation gravimetry. . What is the difference between gravimetric . Precipitation gravimetric analysis separates ions from a solution by using the precipitation process (the reaction that creates an insoluble solid product from the reaction of two soluble solid products). . Electro gravimetric method is employed to separate the ions of a substance, often a metal. In this method, the analyte solution is .
b) Gravimetric precipitation method: Gravimetric precipitation method involves the conversion of an analyte to a sparingly soluble precipitate, which is then filtered and washed to make it free from impurities. Then, it is finally converted to a product of a known composition by suitable heat treatment (ignition). The product obtained is then weighed.
The mass of suspended solids is the difference between the filter’s final mass and its original mass. We call this a direct analysis because . we call the method volatilization gravimetry. In determining the mois-ture content of bread, for example, we use thermal energy to vaporize the . Most precipitation gravimetric methods were .Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate and quantify the analyte of interest. . Two common types include volatilization gravimetry and .Find step-by-step Chemistry solutions and your answer to the following textbook question: Explain the difference between (a) a colloidal and a crystalline precipitate. (b) a gravimetric precipitation method and a gravimetric volatilization method. *(c) precipitation and coprecipitation. (d) peptization and coagulation of a colloid. *(e) occlusion and mixed-crystal . Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. . When the signal is the mass of a precipitate, we call the method precipitation gravimetry.
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 7.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction.Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction. In a direct precipitation gravimetric analysis, . Volatilization methods Another direct volatilization method involves carbonates which generally decompose to release carbon dioxide when acids are used. Because carbon dioxide is easily evolved when heat is applied, its mass is directly established by the measured increase in the mass of the absorbent solid used.
volatilization gravimetry chemistry
volatilization gravimetric method
Four primary gravimetric analysis methods are used to determine an analyte's mass. Outside of precipitation gravimetry, other common methods include volatilization gravimetry, electrogravimetry . As nouns the difference between evaporation and volatilization. is that evaporation is the process of a liquid converting to the gaseous state while volatilization is the conversion of a solid or liquid into a gas; vaporization; evaporation or sublimation.Explain the difference between *(a) a colloidal and a crystalline precipitate. (b) a gravimetric precipitation method and a gravimetric volatilization method. *(c) precipitation and coprecipitation. (d) peptization and coagulation of a colloid. *(e) occlusion and mixed-crystal formation. (f) nucleation and particle growth.
12-1. Explain the difference between*(a) a colloidal and a crystalline precipitate.(b) a gravimetric precipitation method and a gravimetric volatilization method.*(c) precipitation and coprecipitation.(d) peptization and coagulation of a colloid.*(c) occlusion and mixed-crystal formation.(f) nucleation and particle growth. Gravimetric Analysis. Gravimetric analysis is a quantitative method in analytical chemistry wherein the concentration of a substance present in a sample is evaluated based on the measurement of its mass. This is done by precipitating the analyte (substance being analyzed) in a sample as a solid compound which is then separated, washed, dried and weighed.
A gravimetric volatilization method, on the other hand, involves heating a sample to drive off a volatile component, such as water or a volatile compound. The mass loss due to volatilization is then used to determine the concentration of the volatile component in the sample. Step 3/6Gravimetric analysis is a quantitative method for estimating the quantity of a chemical correctly based on the mass of a solid.. Gravimetric analysis can be used in a variety of ways including the chemical analysis of ores and other industrial materials, equipment calibration, and elemental analysis of inorganic substances.. It is used to assess the chemical composition of rocks, .
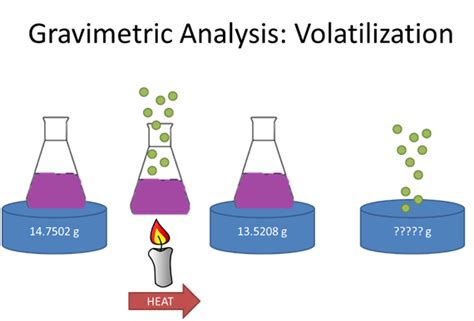
Discuss the differences between precipitation and coprecipitation in gravimetric analysis. Difficulty: Medium. . In contrast, a gravimetric volatilization method involves separating the analyte by converting it to a gas of known chemical composition. The mass of the gas serves as a measure of the analyte concentration.If we then find the difference between these two masses, we get the mass of volatile substances liberated. This simple calculation is the main principle of volatilization gravimetry. Examples of volatile compounds which may be released during chemical or thermal decomposition of a sample include .
In precipitation gravimetry an insoluble compound forms when we add a precipitating reagent, or precipitant, to a solution that contains our analyte.In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate potentially can serve as a gravimetric .Gravimetric analysis works on the idea of determining the mass of an ion in a pure compound and then using that information to calculate the mass percent of that same ion in a known quantity of an impure compound. Precipitation, volatilization, electro-analytical, and various physical methods are the four basic forms of this method of analysis.a) The following are the differences between colloidal and crystalline precipitates: • Size of particles: Colloidal precipitates consist of particles that have a diameter less than which cannot be seen by naked eyes, while crystalline precipitates contain bigger size particles more than • Suitability for gravimetric analysis: Colloidal precipitates are not suitable for gravimetric .
best iphone xr case drop test
WEBfmem A cross-platform library for opening memory-backed libc streams. This library was written for Criterion to implement stringification functions for user-defined types.
difference between gravimetric precipitation and volatilization method|example of volatilization gravimetry